Photo: Matthew Monarca
We updated the USDA Phytoremediation Database. For information on many different soil contaminants and many different plant mechanisms for remediation, please visit the database online.
Lead Phytoremediation
Many gardeners believe that plants like sunflowers can remove harmful contaminants from soil. Findings suggest that they are not effective at removing lead as previously thought.
Exposure to lead causes over 400,000 deaths per year in the US. Lead is not just in paint and pipes; it’s also in soil. For years, plant-lovers have been trying to find a way to remove dangerous lead from soil, and many believe sunflowers and other plants are the silver bullet. Sorry gardeners, but we have some bad news: plants are no match for stubborn lead-filled soil. Why not? The dirty truth is, well, complicated.
Writing in the International Journal of Phytoremediation, Dr. Egendorf, Dr. Cheng and Dr. Groffman from The Graduate Center, Brooklyn College, and the Advanced Science Research Center at the City University of New York, along with Dr. Gerry Moore at the USDA Natural Resource Conservation Service (NRCS) conducted a review to unearth the realities of plant uptake and subsequent removal (‘phytoextraction’) of lead in soil. Dr. Egendorf will be a postdoctoral fellow at the Cornell Atkinson Center for Sustainability this August.
While many plants can almost miraculously remediate soil and water (accomplishing ‘phytoremediation’) for a variety of contaminants like arsenic, nickel, or PAHs—lead is different. The authors discuss why that is, exploring the many factors that make lead immobile in soil and unavailable to plant roots. The authors explain the many mechanisms plants employ to keep lead stored outside or inside their roots, preventing it from moving into above-ground tissues. On top of these particular soil-plant-lead dynamics that make ‘phytoextraction’ not feasible, the authors emphasize a major impediment: there is no consensus on what successful lead-phytoextraction actually looks like.
To address this striking gap, and keep practitioners safe, the authors propose a 3-point rating system to determine lead-phytoextraction success. Using these criteria, they evaluated the existing and updated USDA Phytoremediation database. Having done such a review, the authors emphasize that without using amendments to alter soil (one important criterion to adhere to), “no plant has been shown to be suitable for lead phytoextraction.”
This will be a blow to all faithful believers, from industrious home and community gardeners, to industry practitioners. But there is a bright side: many plants are capable of stabilizing or holding contaminants like lead in place (‘phytostabilization’). The authors urge readers to recognize that “remediation of soil lead is a wide-spread and urgent concern.” While using plants to remove lead does not work, keeping leaded-soil covered is extremely important, and “phystostabilization is an essential tool for this endeavor.” Bottom line: when it comes to quick fixes for remediation of your soil, don’t be mis-lead.
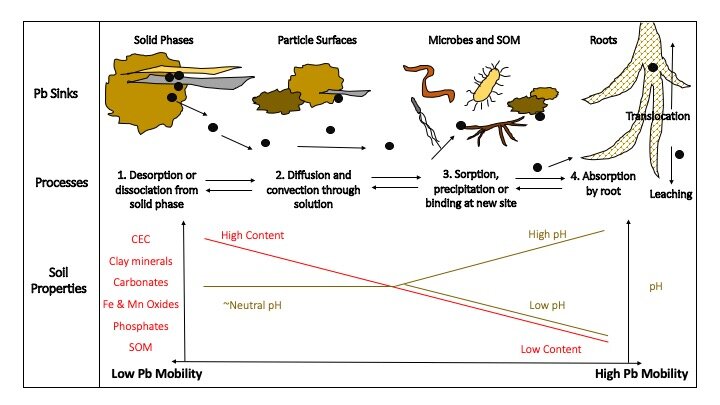
Lead biogeochemistry in soils: relationships between sinks, processes and soil properties.
Lead in soil may be found in four sinks, here represented from least to most mobile or available to plant roots: (1) bound, diffused, occluded or precipitated in solid phases, (2) adsorbed to particle surfaces, (3) complexed with microbes and soil organic matter (SOM), or (4) bound to or absorbed by roots. In order for Pb to be mobilized in soil and enter plant roots, it may undergo four processes moving from least to most mobility: (1) desorption or dissociation from the solid phase, (2) diffusion and convection through soil solution, (3) sorption, precipitation or binding at a new site, and finally, (4) absorption by roots. Mobilized Pb may also be translocated to aboveground biomass (in limited conditions and for limited species) or leached through the soil profile. There are a number of important soil properties that contribute to Pb mobility. As depicted here, high content of CEC, clay minerals, carbonates, Fe and Mn oxides, phosphates, SOM, and neutral pH contribute to low Pb mobility (left side of the diagram). Conversely, low content of these soil properties and alkaline or acidic soils contribute to increased Pb mobility (right side of the diagram).